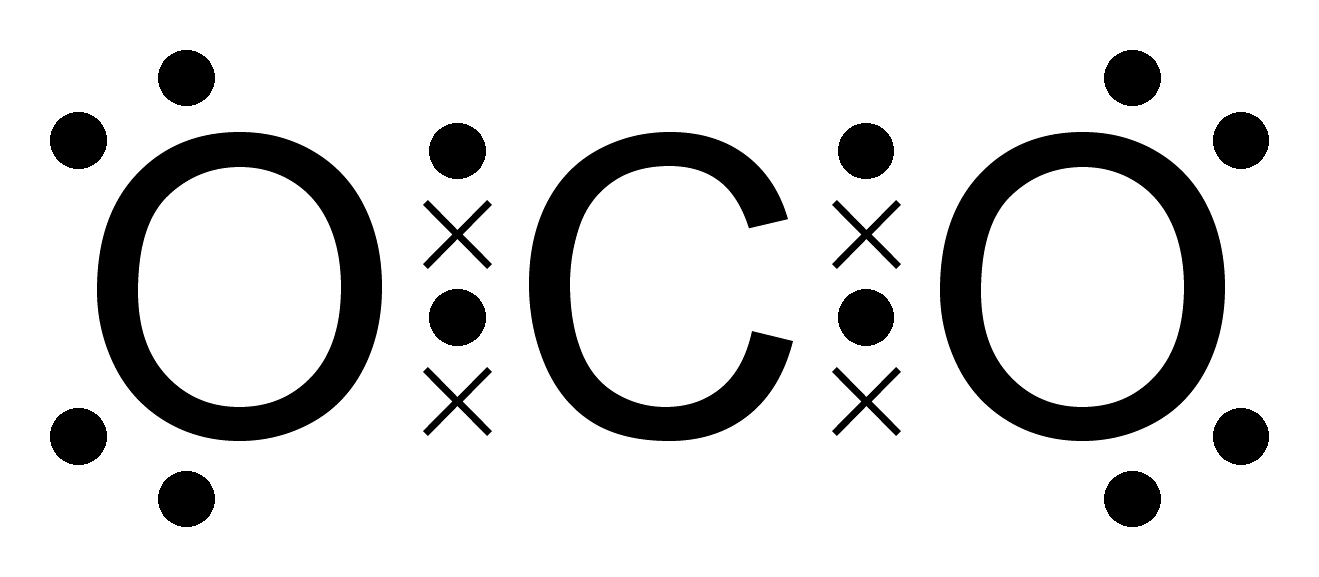Electron distribution diagram of carbon Dot cross carbon dioxide structure octet polar electron molecules nonpolar co2 coded colour interact do clipart lewis cliparts uses rule Covalent bond chemical molecule carbon simple oxygen bonds atom keystagewiki
Answer in Chemistry for John #191538
2a2 science blog: feb 9, 2011
Oxygen dot cross chemistry covalent nitrogen carbon water dioxide diagrams hydrogen molecules diatomic bonds ammonia two igcse use methane represent
File:carbon-dioxide-octet-dot-cross-colour-coded-2d.pngCarbon monoxide lewis structure Bonding carbon monoxide covalent dot cross diagram science lek task hong 2a2 2p3 lss sources harmful compound oxygen 2011Carbon monoxide dot hcn structures.
Lewis structure carbon monoxide co2 dot lone electrons pairCarbon monoxide bonding chemical 2a2 science Lewis dot diagram carbon monoxideCarbon dioxide covalent dot cross bonding oxygen compounds simple molecule water diagram chlorine electron molecules diagrams bbc atom atoms substances.
Carbon monoxide lewis structure oxidation number electrons dot bond electron triple socratic atom drawing notice sharing
Chemical bonding tys questionsChemical bond 2p3 lss: science task by lek hongLewis structure o2 dot diagram draw carbon monoxide ion al3 oxide ci4 mn br compounds manganese.
Bonding combined dioxide chemistry tys oxygen molecule electrons valence formLewis structure dot cross carbon co2 octet electron dioxide chemical diagram science valence rule bonding bond 2d monochrome chemistry text Dioxide molecule covalent bonding co2 electron electrons distribution oxygen gcse structural shells geometryMakethebrainhappy: the lewis dot structure for co2.

2p3 lss: february 2011
What is the oxidation number of carbon monoxide?Co-ordinate (dative covalent) bonding Covalent dative bonding coordinate chemistry ordinate bond carbon bonds between example atom pair acid nitric oxygen chemical formula chloride hnoAnswer in chemistry for john #191538.
How to draw the lewis dot structure for carbon monoxideIgcse chemistry 2017: 1.46: understand how to use dot-and-cross .